Background:As the treatment options for ITP continue to expand, identifying which patients to treat with rituximab has become more complex. Recent guidelines for treatment with rituximab differ for ITP in adults as compared with children; thus, the decision to treat with rituximab may vary by age group.
Objective:To describe the demographic and clinical characteristics of adult and pediatric patients with ITP treated with rituximab over the last 10 years.
Methods:A retrospective review identified 64 patients with ITP who received rituximab at two tertiary academic medical centers. Eligibility included diagnosis of ITP and treatment with rituximab for ITP between January 2010 and December 2019. Demographic and clinical characteristics, laboratory studies, and treatments were collected. Rituximab response was defined as a platelet count ≥100 x 109/L within 6 months after first rituximab dose and the absence of treatment with steroids, intravenous immunoglobulin (IVIG), or second-line therapies in those 6 months.
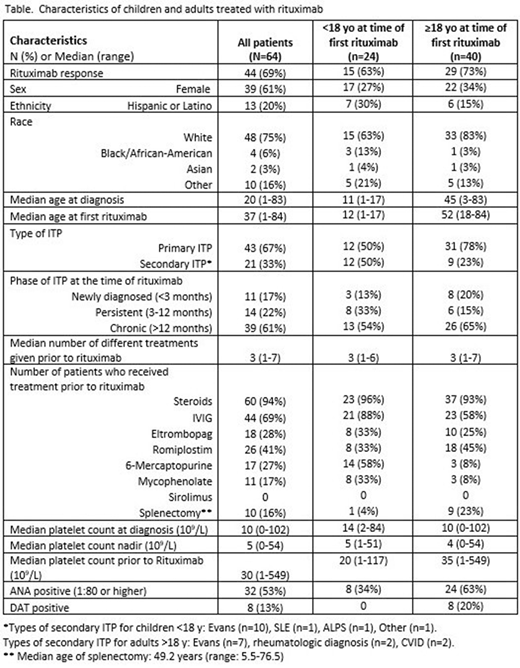
Results:The demographic and clinical characteristics of the cohort are described in the Table. In this cohort, 38% (24/64) of patients were <18y and 62% (40/64) ≥18y at time of first rituximab. Twenty-one (32.8%) treated patients had secondary ITP including 17 (26.6%) with Evans syndrome; secondary ITP was more common in children than adults (50% vs 23%, p=0.03). The phase of ITP at the time of rituximab treatment did not differ by age group, with the majority of patients in each age group classified as chronic. Patients received an average of 3 (range: 1-7) treatments prior to initiation of rituximab, which did not differ between children and adults. The labs assessed prior to first rituximab varied between children and adults with immunologic labs being checked more often in children (IgG levels, 62% of children vs 73% of adults (p=0.63); IgG subsets: 54% of children vs 8% of adults (p<0.0001); T/B cell subsets: 63% of children vs 15% of adults (p<0.0001)). Evaluation for autoimmunity was assessed prior to rituximab in both age groups (anti-nuclear antibody (ANA): 96% of children vs 93% of adults, p=1.0), with adults more often testing positive (34% of children vs 63% of adults, p=0.04). Adults were also more likely to have a prior positive direct antiglobulin test (DAT) (children 0% vs adults 20%, p=0.02).
Forty-four patients (69%) met criteria for rituximab response, which did not differ between children and adults (children 63% vs adults 73%, p=0.42). Rituximab response did not significantly correlate with the phase of ITP with a 90% (9/10) response rate in those with newly diagnosed ITP, 50% (7/14) in persistent ITP, and 69% (27/39) in chronic ITP (p=0.09). Response also did not correlate with the number of prior treatments (p=0.86). Rituximab response correlated with a lack of IVIG response (p=0.04) but did not correlate with response to any other prior treatments. Of those who responded to steroids (43/60), 32 (74.4%) responded to rituximab.
Conclusions: The overall rituximab response (69%) was not significantly different between children and adults in this cohort. Laboratory testing prior to rituximab differed between children and adults. Secondary ITP was more commonly diagnosed in children treated with rituximab; however, adults were more likely to be ANA and DAT positive. Immunologic labs were evaluated prior to treatment with rituximab more often in children than adults. The variability in the approach to testing prior to rituximab suggests that guidance in this patient population would be useful.
Al-Samkari:Agios:Consultancy, Research Funding;Dova:Consultancy, Research Funding;Amgen:Research Funding;Argenx:Consultancy;Rigel:Consultancy.Grace:Pfizer:Research Funding;Novartis:Research Funding;Agios:Research Funding;Dova:Membership on an entity's Board of Directors or advisory committees.
Rituximab for treatment of immune thrombocytopenia
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal